The new way to be stone-free.


Complete removal of kidney stones with mediNiK®.
-
Effective retrieval of stone fragments
-
Lower recurrence rate
-
Shorter operation time
The two-component solution
mediNiK®, the innovative 2-component hydrogel with ready-to-use sterile syringes, increases the effectiveness of endoscopic kidney stone removal:
Base substance
The base substance encloses the stone fragments.

ACTIVATOR
When the activator is injected, a soft gel forms within a few seconds.
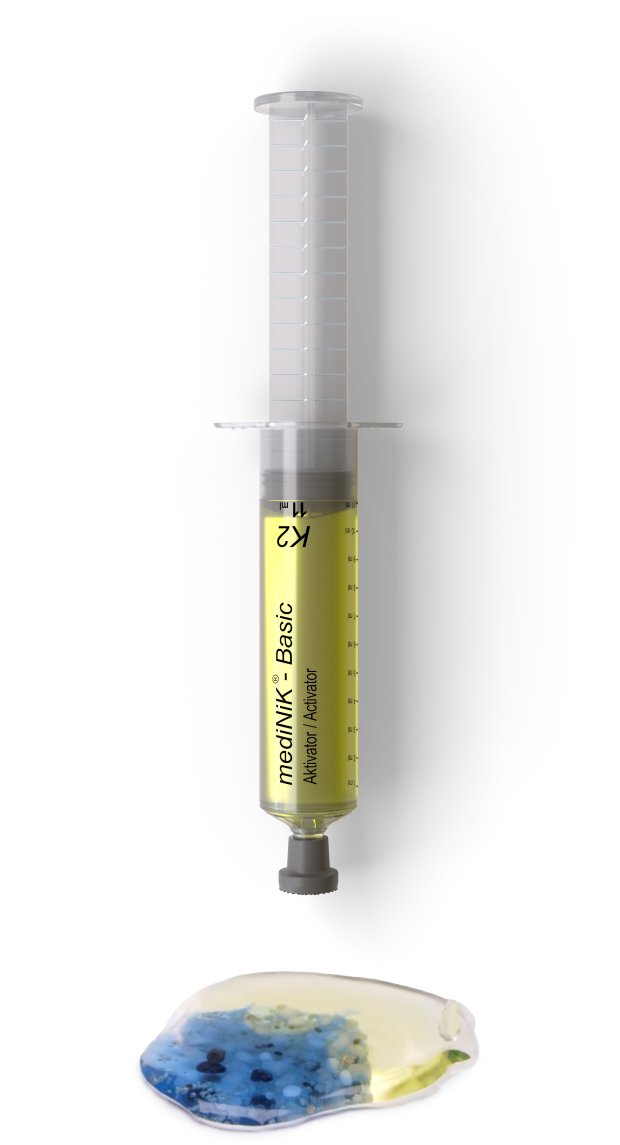
Hydrogel mediNiK®
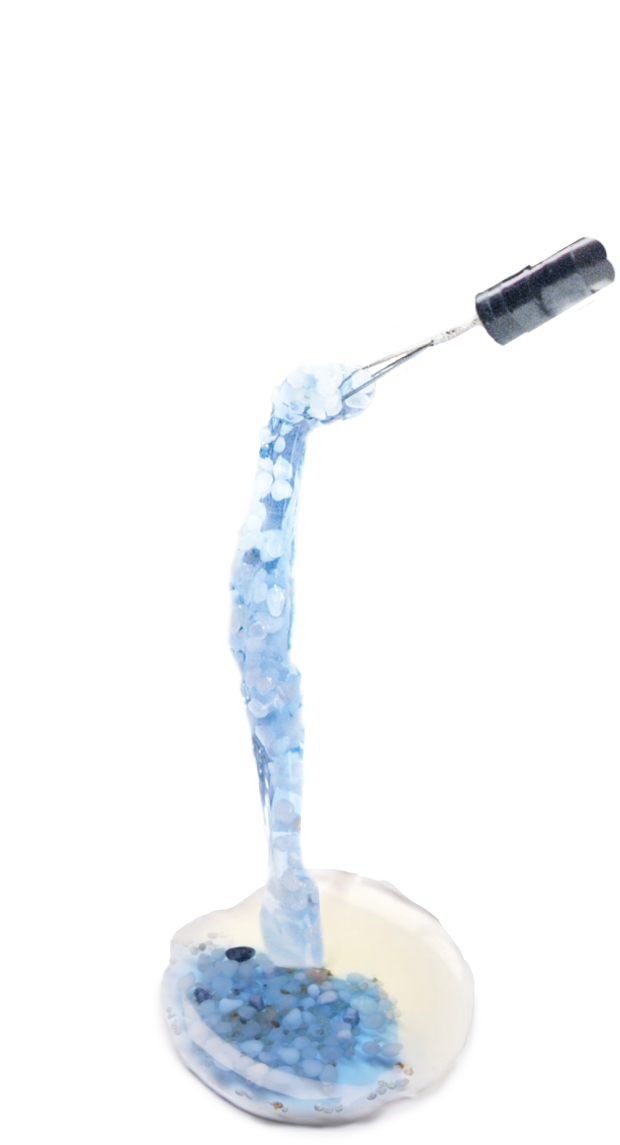
The resulting hydrogel with the stone fragments can now be removed by a grasping instrument without the gel sticking to instruments or tissue.
Base substance
The base substance encloses the stone fragments.

Activator
When the activator is injected, a soft gel forms within a few seconds.

Hydrogel mediNiK®
The resulting hydrogel with the stone fragments can now be removed by a grasping instrument without the gel sticking to instruments or tissue.

The mediNiK® hydrogel method in use
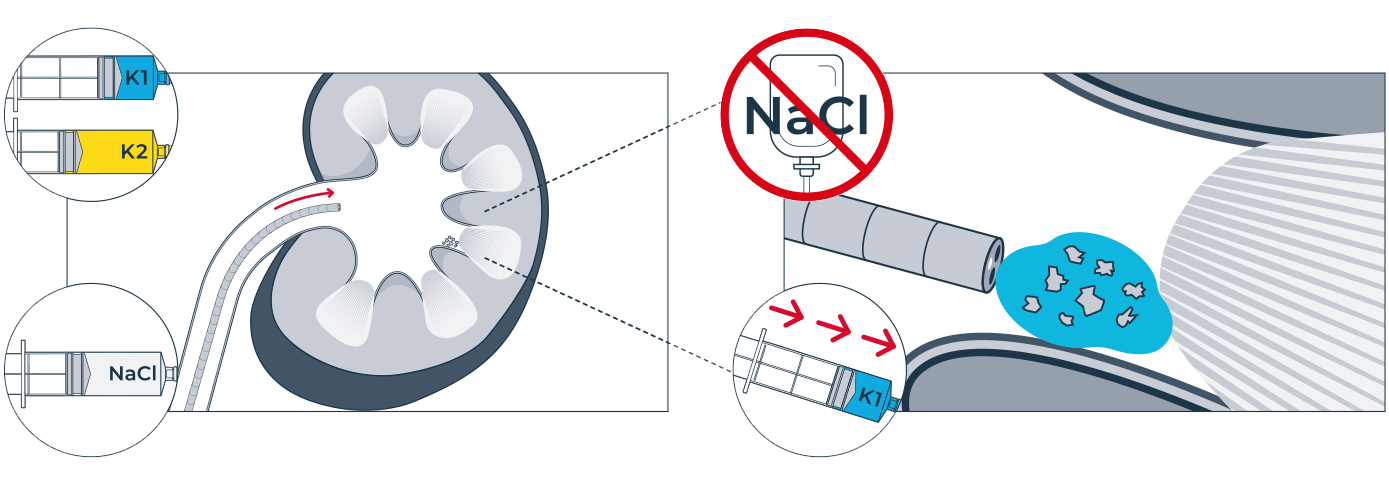
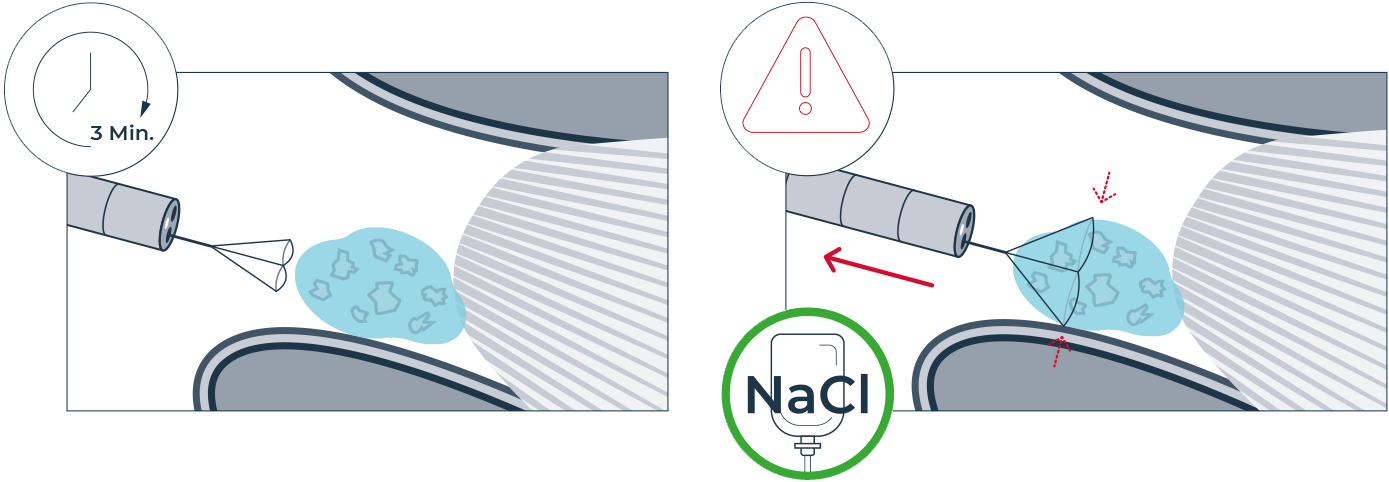
One of the challenges of kidney stone removal is to remove the smallest kidney stone fragments and achieve 100% stone clearance. Now there is a solution: mediNiK®, a medical hydrogel for removing kidney stone fragments as part of flexible ureterorenoscopy. The innovative two-component mediNiK® hydrogel does not adhere to surgical instruments or the sensitive renal mucosa, is biocompatible and makes it possible to effectively remove even the smallest kidney stone fragments (< 1 mm). When using mediNiK®, the two components of the hydrogel are injected in succession via the working channel of the endoscope.
PHASE 2 Hydrogel formation
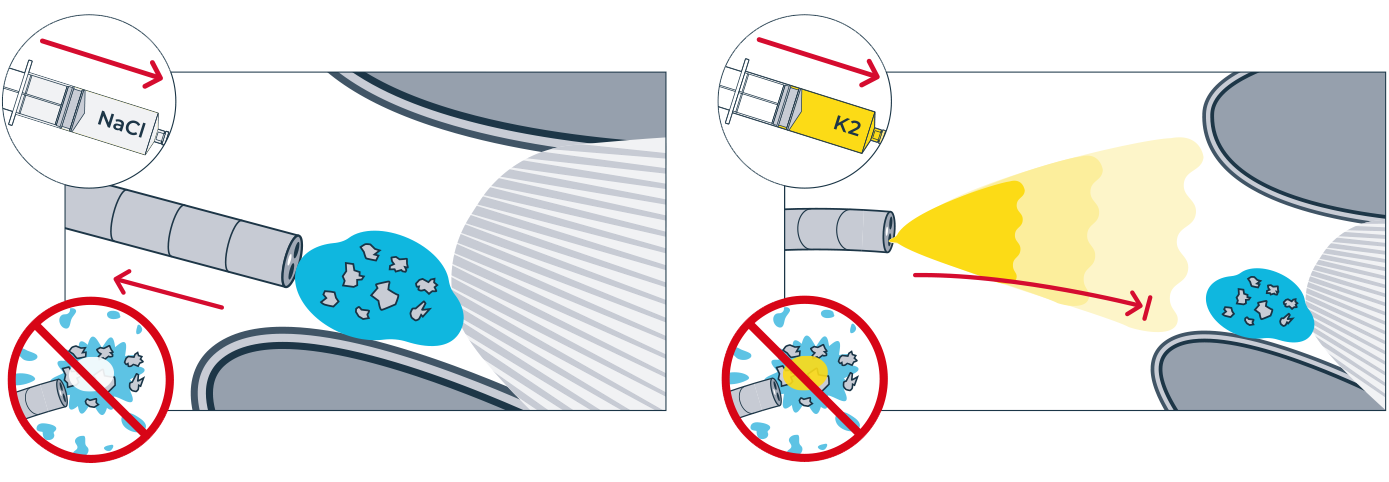
Injection of the yellow component (K2) of the hydrogel as an activator.
PHASE 3 Retrieval
The hydrogel clot is carefully removed with the aid of an endoscopic grasper.
Medical Need
Current grasping instruments and extraction baskets used in ureteroscopic stone removal (URS) are not able to efficiently remove small fragments, especially those smaller than 1 mm. This often means that complete freedom from stones is not achieved. The fragments that remain after interventional therapy are referred to as residual fragments. These can lead to further stone formation, cause persistent urinary tract infections or trigger colic if they are displaced into the ureter.
The risk of disease recurrence within five years of the intervention is 21-59% in patients with residual fragments. The risk of recurrence is increased particularly in patients with struvite stones, also known as infectious stones, as these are often caused by urinary tract infections.
Residual fragments of any size can be clinically relevant. Studies show that residual fragments smaller than 4 mm are just as clinically relevant as larger fragments. The smaller the residual fragments are, the greater the probability that they will be passed spontaneously. However, there is evidence that even fragments less than 1 mm in size, also known as dust, do not always pass spontaneously and may enlarge. There is evidence that there is a higher risk of recurrence of the disease in patients with residual fragments less than 1 mm compared to patients who are completely stone-free. mediNiK® hydrogel can be used to remove the smallest kidney stone fragments to meet the medical need for 100% stone clearance.
HOW EXPERTS ASSESS mediNiK®

“The new mediNiK® hydrogel method takes us to a new level of stone freedom.”

“With mediNiK® I can guarantee to my patients that they will be stone-free after an operation.”

“For interventional urinary stone therapy, it is the most innovative concept in 20 years for achieving stone clearance after lithotripsy.”

“With mediNiK® the path to absolute freedom from stones is within reach and represents a major milestone in urology.”
Prof. Andreas Neisius MD
Deputy Medical Director, Head of Urology and Pediatric Urology/Head of Prostate Cancer Center, Hospital of the Brothers of Mercy, Trier
Medical director Head of Endourology and Urinary Stone Center, Klinikum rechts der Isar, Munich
Christopher Netsch MD
Urology consultant, Asklepios Clinic Barmbek, Hamburg
Thomas Amiel MD
Urology consultant, Endourology and Urinary Stone Center, Klinikum rechts der Isar, Munich
WIE EXPERTEN mediNiK® BEWERTEN

“The new mediNiK® hydrogel method takes us to a new level of stone freedom.”
Prof. Andreas Neisius MD
Deputy Medical Director, Head of Urology and Pediatric Urology/Head of Prostate Cancer Center, Hospital of the Brothers of Mercy, Trier

“With mediNiK® I can guarantee to my patients that they will be stone-free after an operation.”
Michael Straub MD
Medical director Head of Endourology and Urinary Stone Center, Klinikum rechts der Isar, Munich

“For interventional urinary stone therapy, it is the most innovative concept in 20 years for achieving stone clearance after lithotripsy.”
Christopher Netsch MD
Urology consultant, Asklepios Clinic Barmbek, Hamburg

“With mediNiK® the path to absolute freedom from stones is within reach and represents a major milestone in urology.”
Thomas Amiel MD
Urology consultant, Endourology and Urinary Stone Center, Klinikum rechts der Isar, Munich
The founders
Purenum GmbH was founded at the end of 2017 as a spin-off of the Fraunhofer Institute for Manufacturing Technology and Materials Research IFAM in Bremen. The name Purenum is derived from the Latin "purum renum" (the pure kidney). The company specializes in the development of medical adhesives and hydrogels.
Purenum's first product is to be a hydrogel. It is intended to combine small, previously non-graspable kidney stone fragments after fragmentation. These can then be removed from the kidney using a conventional grasper. The idea arose from the research of a young urologist. He was looking for a "kidney stone adhesive" and found a book on biological and bio-mimetic adhesives. The co-editor, Dr Ingo Grunwald, did not have a ready-made adhesive, but he did have a promising idea.
Three months later, Grunwald developed a promising solution. Together with his colleague Manfred Peschka and the urologist, he submitted a successful research proposal to the BMBF funding programme GO-Bio ("Biotechnology Start-up Initiative"). The "mediNiK - adhesive for removing kidney stone fragments" project was launched. Grunwald's team researched the basics up to the design freeze.
At the same time, Peschka developed a viable business plan. This enabled the search for investors and the founding of Purenum. The company also received funding from the BMBF for another project: "mediGLUE - Development of innovative biomimetic adhesives for medical applications". In addition to outstanding questions about mediNiK, this project is also investigating the basis for an adhesive for fixing bone splinters, particularly in joint bones.
The mediNiK product was certified in May 2021. Since then, it can be used in patients throughout Europe. In June 2023, Purenum GmbH became a subsidiary of FARCO-PHARMA GmbH.
Made in Germany
We develop products that make everyday life easier for healthcare professionals and patients. That is why our quality standards are unconditional and uncompromising. In order to set the quality standards in the market, we produce exclusively in Germany according to the highest pharmaceutical standards. And can thus give our promise of quality “Made in Germany”:
Do you have any questions?
What is the proven benefit of the product for the patient?
By removing significantly more small renal stone fragments (< 1 mm) than conventional URS without the use of mediNiK®, mediNiK reduces the risk of recurrence for patients if recrystallization nuclei are no longer present. Nearly all kidney stones < 1 mm are a potential nucleus for new, larger renal stones.
What is the advantage of mediNiK® compared to the blood clot method?
mediNiK® has three main advantages over the use of autologous blood. Firstly, the high-contrast color of mediNiK® enables more precise injection and thus secure/complete integration of the stone fragments. Secondly, gel formation occurs much faster than blood clotting. And thirdly, any gel residue remaining in the kidney after the procedure dissolves as a result of diuresis and cannot cause an obstruction.